Publikationen
- [95] "Insights into Bimetallic CoFe Nanoparticles Incorporated on N-Doped Carbon Nanosheets toward Acidic Oxygen Reduction Reaction"1 | Hariprasad Ranganathan, Hossein Bemana, Alexandre Terry, Shuai Chen, Nikolay Kornienko | energyfuels - online - Aug 2025
- [94] "Electrochemical Transformation of Copper Sulfide Electrodes for Selective CO2-to-Formate Conversion"2 | Roser Fernández-Climent, Daniele Giusi, Matteo Miceli, Camilo A. Mesa, Ana Gutiérrez-Blanco, Junnan Li, Jesús Redondo, Frederik Schiller, Sara Barja, Veronica Celorrio, Nikolay Kornienko, Claudio Ampelli, Sixto Giménez | ChemSusChem - online - Aug 2025
- [93] "Antimony and Bismuth Complexes as Visible Light Photosensitizers in Catalytic Oxidation Reactions "3 | André Korzun, Morgan J. McKee, Hagen Neugebauer, Ori Green, Gregor Schnakenburg, Stefan Grimme, Nikolay Kornienko, Alessandro Bismuto | Inorganic Chemistry - vol 64, issue 30, 15667-15679 - Jul 2025
- [92*] "Accelerating lithium-mediated nitrogen reduction through an integrated palladium membrane hydrogenation reactor"4 | Hossein Bemana, Hendrik Schumann, Morgan McKee, Senada Nozinovic, Jörg Daniels, Ralf Weisbarth, Nikolay Kornienko | Nature Communications - vol 16 - Jul 2025
- [91] "Metal-like Behavior of a 2D Molecular Catalyst Enables Redox-Decoupled Electrocatalysis"5 | Yang Wang, Dongyu Zhang, Ting Chen, Caijie Su, Yi Xie, Changzheng Wu, Nikolay Kornienko | National Science Review - vol 12, issue 8 - Aug 2025
- [90*] "Dynamic active sites behind Cu-based electrocatalysts: Original or restructuring-induced catalytic activity"6 | Shuai Chen, Farzaneh Farzinpour, Nikolay Kornienko | Chem - online - May 2025
- [89] "Impact of Co2+ substitution by Fe2+ on the thermal behavior of a hydrated fluoride, precursor of a mixed iron-based oxyfluoride with reduced cobalt content as efficient OER electrocatalyst"7 | Alexandre Terry, Guillaume Duval, Samuel Mathiot, Jean-Marc Grenèche, Ralf Weisbarth, Edouard Boivin, Vincent Maisonneuve, Annie Hémon-Ribaud, Nikolay Kornienko, Amandine Guiet, Jérôme Lhoste | Comptes Rendus. Chimie - vol 28, pp 289-300 - Mar 2025
- [88*] "Best practices for in-situ and operando techniques within electrocatalytic systems"8 | Aditya Prajapati, Christopher Hahn, Inez M. Weidinger, Yanmei Shi, Yonghyuk Lee, Anastassia N. Alexandrova, David Thompson, Simon R. Bare, Shuai Chen, Shuai Yan, Nikolay Kornienko | Nature Communications - vol 16 - Feb 2025
- [87] "Two-dimensional nanomaterials for environmental catalysis roadmap towards 2030"9 | Jing Guo, Jianzhong Ma, Junli Liu, Guanjie Huang, Xiaoting Zhou, Francesco Parrino, Riccardo Ceccato, Leonardo Palmisano, Boon-Junn Ng, Lutfi Kurnianditia Putri, Huaxing Li, Rongjie Li, Gang Liu, Yang Wang, Nikolay Kornienko, Shan-Shan Zhu, Zhenwei Zhang, Xiaoming Liu, Nur Atika Nikma Dahlan, Siang-Piao Chai, Jianmin Ma | Chinese Chemical Letters - vol 36, issue 9 - Feb 2025
- [86] "Insights into the Electrochemical Oxidation and Reduction of Nickel Oxide Surfaces"10 | Wenyu Sun, Nitish Govindarajan, Aditya Prajapati, Jiayi Huang, Hossein Bemana, Jeremy T. Feaster, Sneha A. Akhade, Nikolay Kornienko, Christopher Hahn | Applied Materials & Interfaces - vol 17, issue 1 - Jan 2025
- [85] "Total Electrosynthesis of N, N-Dimethylformamide From CO2 and NO3- "11 | Shuai Yan, Shuai Chen, Morgan McKee, Alexandre Terry, Ralf Weisbarth, Nikolay Kornienko | Advanced Science - vol 121, issue 2 - Nov 2024
- [84] "Unlocking C–C cleavage in the electrochemical toolbox"12 | N. Kornienko | Nature Catalysis - vol 7, issue 9, 957–958 - Sep 2024
- [83] "Terpyridine-Decorated Polymer Nanosphere Latex: Template Nanocarriers for the Synthesis of Cu–CeO2 Hollow Spheres"13 | F. Francois, Q. Hy T. Piogé, N. Kornienko, V. Maisonneuve, J. Lhoste, A. Guiet* S. Pascual* | Applied Materials & Interfaces - vol 16, issue 31, 40311-41720 - Aug 2024
- [82] "Mechanistic Insights into the Electrochemical Oxidation of 5-Hydroxymethylfurfural on a Thin-Film Ni Anode"14 A. Prajapati, N. Govindarajan, W. Sun, J. Huang, H. Bemana, J. T. Feaster, S. A. Akhade, N. Kornienko, and Christopher Hahn | ACS Catalysis - vol 14, issue 13, 9640-10417 - Jul 2024
- [81] "Dimethylphosphite Electrosynthesis from Inorganic Phosphorus Building Blocks via Oxidative Coupling"15 J. Li, H. Bemana, N Kornienko | RSC Sustainability - vol 2, issue 8, 2289-2294 - Aug 2024
- [80] "Simple and Scalable Synthetic Route for Tunable Compositions of Multimetallic Oxyfluorides as Oxygen Evolution Reaction Catalysts"16 A Terry, S Mathiot, A Guiet, E Boivin, Z Gohari-Bajestani, V Maisonneuve, A Hémon-Ribaud, R Moury*, N Kornienko, and J Lhoste | ACS Applied Energy Materials - vol 7, issue 24, 11466-11474 - Jun 2024
- [79] "Copper nanoclusters: Selective CO2 to methane conversion beyond 1 A/cm²17" M. Salehi, H.Al-Mahayni, A. Farzi, M. McKee, S. Kaviani, E. Pajootan, R. Lin, N. Kornienko, A. Seifitokaldani | Applied Catalysis B - vol 353 - Sep 2024
- [78] "Enabling epoxidation and oxygen atom transfer via leveraging the water oxidation pathway"18 A. Terry, N. Kornienko | Chem Catalysis - vol 4, issue 3 - Mar 2024
- [77] "Combined Electrochemical and Spectroscopic Investigations of Carbonate-Mediated Water Oxidation to Peroxide"19 H. Bemana, N. Kornienko | iScience - vol 27, issue 4 - Apr 2024
- [76*] "Heterogeneous electrosynthesis of C–N, C–S and C–P products using CO2 as a building block"20 J. Li, H. Heidarpour, G. Gao, M. McKee, H. Bemana, Y. Zhang, C. T. Dinh, A. Seifitokaldani, and N. Kornienko | Nature Synthesis - vol 3, issue 7, 809-824 - Jul 2024
- [75] "Electrocatalysis with Molecules and Molecular Assemblies within Gas Diffusion Electrodes"21 H. Bemana, M. McKee, N. Kornienko | Chemical Science - vol14, issue 47, 13696-13712 - Dec 2023
- [74] "Highly Durable Nanoporous Cu2-xS Films for Efficient Hydrogen Evolution Electrocatalysis under Mild pH Conditions"22 R. Fernández-Climent, J. Redondo, M.García-Tecedor, M. C. Spadaro, J. Li, D. Chartrand, F. Schiller, J. Pazos, M. F. Hurtado, V. de la Peña O’Shea, N. Kornienko, J. Arbiol, S. Barja*, C. A. Mesa, and S. Giménez | ACS Catalysis - vol 13, issue 15 - Aug 2023
- [73*] "Hydrophobic assembly of molecular catalysts at the gas–liquid–solid interface drives highly selective CO2 electromethanation"23 M. McKee, M. Kutter, D. Lentz, M. Kuehnel, N. Kornienko | ChemRxiv - preprint - Feb 2023, Nature Chemistry - vol 17, issue 1, 92-100 - Jan 2025
- [72] "Oxy-reductive C-N bond formation via pulsed electrolysis24" Y. Zhang, H. Al-Mahayni, P. Aguiar, D. Chartrand, M. McKee, A.Seifitokaldani, N. Kornienko | ChemRxiv - preprint - Dec 2022, Nature Chemistry - in. rev. (2023)
- [71] "Feeling the Weight"25 N. Kornienko | Nature Catalysis - vol 5, issue 10, 852-853 - Oct 2022
- [70] "Reversible transition of an amorphous Cu-Al oxyfluoride into a highly active electrocatalyst for NO3− reduction to NH3"26 Amandine Guiet, Alexandre Simonin, Hossein Bemana, Hasan Al-Mahayni, Junnan Li, Kiran Kuruvinashetti, Romain Moury, Annie Hémon-Ribaud, Daniel Chartrand, Vincent Maisonneuve, Jérôme Lhoste, Ali Seifitokaldani, Dominic Rochefort, Nikolay Kornienko | Chem Catalysis - vol 3, issue 5 - May 2023
- [69*] "Electrochemical Formation of C-S Bonds from CO2 and Small Molecule Sulfur Species27" J. Li, H. Al-Mahayani, D. Chartrand, A. Seifitokaldani, N. Kornienko | Nature Synthesis - vol 2, issue 8 - Aug 2023
- [68*] "Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis"28 J. Li, Y. Zhang, K. Kuruvinashetti, N. Kornienko | Nature Reviews Chemistry - vol 6, issue 5, 303-319 - May 2022
- [67] "Emerging opportunities with metal-organic framework electrosynthetic platforms"29 K. Kuruvinashetti, J. Li, Y. Zhang, H. Bemana, M. McKee, N. Kornienko | Chemical Physics Reviews - vol 3, issue 2 - Jun 2022
- [66] "A Super Basic Strategy"30 Y. Zhang, N. Kornienko | Joule - vol 6, issue 1, 32-34 - Jan 2022
- [65] "Strategies for heterogeneous small-molecule electrosynthesis"31 Y. Zhang, J. Li, N. Kornienko | Cell Reports Physical Science - vol 2, issue 12 - Dec 2021
- [64] "Linker-modulated peroxide electrosynthesis using metal-organic nanosheets"32 K. Kuruvinashetti, N. Kornienko | ChemElectroChem - vol 9, issue 10 - May 2022
- [63] "Electrocatalytic Carbon Dioxide Reduction in Acid"33 J. Li, N. Kornienko | Chem Catalysis - vol 2, issue 1, 29-38 - Jan 2022
- [62] "Highly efficient water oxidation via a bimolecular reaction mechanism on rutile structured mixed-metal oxyfluorides"34 Z. Gohari-Bajestani, X.Wang, A. Guiet, R. Moury, J.-M. Grenèche, A.Hémon-Ribaud, Y. Zhang, D. Chartrand, V. Maisonneuve, A. Seifitokaldani, N. Kornienko, J. Lhoste | Chem Catalysis - vol 2, issue 5, 1114-1127 - May 2022
- [61] "Adaptive framework CO2 catalysis"35 N. Kornienko | Chem - vol 7, issue 10 - Oct 2021
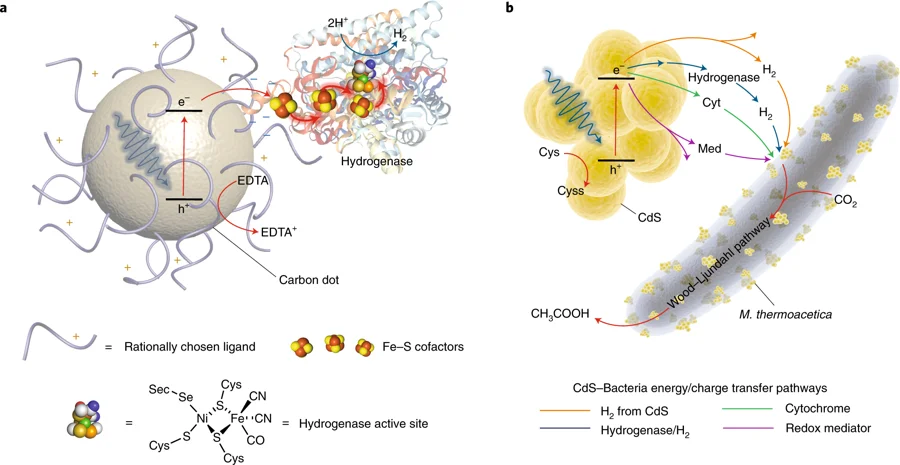
- [60] "Pushing the methodological envelope in understanding the photo/electrosynthetic materials-microorganism interface"36 K. Kuruvinashetti, N. Kornienko | iScience - vol 24, issue 9 - Sep 2021
- [59] "C-N triple bond cleavage via trans-membrane hydrogenation"37 Y. Zhang, N. Kornienko | Chem Catalysis - vol 2, issue 3, 499-507 - Mar 2022
- [58] "Conductive metal-organic frameworks bearing M-O4 active sites as highly active biomass valorization electrocatalysts"38 Y. Zhang, N. Kornienko | ChemSusChem - vol 15, issue 13 - Jul 2022
- [57] "Electrochemically Driven C-N Bond Formation from CO2 and Ammonia at the Triple-Phase Boundary"39 J. Li, N. Kornienko | Chemical Science - vol 13, issue 14, 3957-3964 - Apr 2022
- [56] "Probing electrosynthetic reactions with furfural on copper surfaces"40 J. Li, N. Kornienko | Chemical Communications - vol 57, issue 42, 5127-5130 - May 2021
- [55] "Towards atomic precision in HMF and methane oxidation electrocatalysts"41 Y. Zhang, J. Li, N. Kornienko | Chemical Commununications - vol 57, issue 35, 4230-4238 - May 2021
- [54] "Rational incorporation of defects within metal-organic frameworks generates highly active electrocatalytic sites"42 N. Heidary, D. Chartrand, A. Guiet, N. Kornienko | Chemical Science - vol 12, issue 21, 7324-7333 - Jun 2021
- [53] "Amorphous iron-manganese oxyfluorides, promising catalysts for Oxygen Evolution Reaction under acidic media"43 K. Lemoine, Z. Gohari-Bajestani, R. Moury, A. Terry; A. Guiet, J.-M. Grenèche, A. Hémon-Ribaud, Annie, N. Heidary, V. Maisonneuve, N. Kornienko, J. Lhoste | Applied Energy Materials - vol 4, issue 2, 1173-1181 - Feb 2021
- [52] "Operando spectroscopy of nanoscopic metal/covalent organic framework electrocatalysts"44 N. Kornienko | Nanoscale - vol 13, issue 3, 1507-1514 - Jan 2021
- [51] "Shell Isolated Nanoparticle Enhanced Raman Spectroscopy for Renewable Energy Electrocatalysis"45 K. Kuruvinashetti , Y. Zhang , J. Li, N. Kornienko | New Journal of Chemistry - vol 44, issue 46, 19953-19960 - Dec 2020
- [50] "Metal-based nanomaterials for efficient CO2 electroreduction: Recent advances in mechanism, material design, and selectivity"46 V. C. Hoang, V. Gomes, N. Kornienko | Nano Energy - vol 78, 105311 - Dec 2020
- [49] "Speeding up Nanoscience and Nanotechnology with Ultrafast Plasmonics"47 N. Maccaferri, S. Meuret, N. Kornienko, D. Jariwala | Nano Letters - vol 20, issue 8, 5593-5596 - Aug 2020
- [48] "Mechanochemical synthesis of cobalt/copper fluorophosphate generates a multifunctional electrocatalyst"48 K. Lemoine N. Heidary, Y. Inaguma, N. Kornienko | Chemical Communications - vol 56, issue 65, 9276-9279 - Aug 2020
- [47*] "Electrochemically Triggered Dynamics Within a Hybrid Metal-Organic Electrocatalyst"49 N. Heidary, M. Morency, D. Chartrand, K. H. Ly, R. Iftimie, N. Kornienko | J. Am. Chem. Soc. In Press (2020)
- [46] "Operando Vibrational Spectroscopy for Electrochemical Biomass Valorization"50 N. Heidary, N. Kornienko | Chemical Communications - vol 56, issue 62, 8726-8734 - Aug 2020
- [45] "A One-Pot Route to Faceted FePt-Fe3O4 Dumbbells: Probing Morphology–Catalytic Activity Effects in O2 Reduction Catalysis"51 K. J. Jenkinson, A. Wagner, N. Kornienko, E. Reisner, A. E. H. Wheatley | Advanced Functional Maters - vol 30, issue 25, 2002633 - Jun 2020
- [44] "Surface Chemistry Modulates CO2 Reduction Reaction Intermediates on Silver Nanoparticle Electrocatalysts"52 T.G.A.A. Harris, D. Chartrand, N. Heidary, L. Prado-Perez, K. H. Ly, N. Kornienko | ChemRxiv - preprint - Mar 2020
- [43] "Disparity of cytochrome utilization in anodic and cathodic extracellular electron transfer pathways of Geobacter sulfurreducens biofilms"53 N. Heidary, N. Kornienko, S. Kalathil, X. Fang, K. H. Ly, H. F. Greer, E. Reisner | JACS - vol 142, issue 11, 5194–5203 - Mar 2020
- [42] "Heterogeneous Electrocatalytic Reduction of CO2 Promoted by Secondary Coordination Sphere Effects"54 J. Li, Y. Zhang, N. Kornienko | New Journal of Chemistry - vol 44, issue 11, 4246-4252 - Mar 2020
- [41] "Electrochemical Biomass Valorization on Gold-Metal Oxide Nanoscale Heterojunctions Enables Investigation of both Catalyst and Reaction Dynamics with Operando Surface-Enhanced Raman spectroscopy"55 N. Heidary, N. Kornienko | Chemical Science - vol 11, issue 7, 1798-1806 - Feb 2020
- [40] "Host-guest Chemistry Meets Electrocatalysis: Cucurbit[6]uril on a Au Surface as Hybrid System in CO2 Reduction"56 A.Wagner, K. H. Ly, N. Heidary I. Szabo T. Foeldes, K. I. Assaf , S. J. Barrow, K. Sokolowski, M. Al-Hada, N. Kornienko, M. F. Kuehnel, E. Rosta, I. Zebgerm W. M. Nau, O. A. Scherman, E. Reisner | ACS Catalysis - vol 10, issue 1, 751-761 - Jan 2020
- [39] "2020 roadmap on two-dimensional nanomaterials for environmental catalysis"57 Y. Yang, M. Wu, X. Zhu, H. Xu, Si Ma, Y. Zhi, H. Xia, X. Liu, J. Pan, J.-Y. Tang, S.-P. Chai, L. Palmisano, F. Parrino, K. Liu, J. Ma, Z.-L. Wang, L. Tan, Y.-F. Zhao, Y.-F. Song, P. Singh, P. Raizada, D. Jiang, Di Li, RA Geioushy, J.Ma, K. Zhang, S. Hu, R. Feng, G. Liu, M. Liu, Z. Li, M. Shao, N. Li, J. Peng, W.-J. Ong, N. Kornienko, Z. Xing, X. Fan, J. Ma. | Chinese Chemical Letters - vol 30, issue 12, 2065-2088 - Dec 2019
- [38] "Investigation of mixed-metal (oxy)fluorides as a new class of water oxidation electrocatalysts"58 K. Lemoine, J. Lhoste, A. Ribaud, N. Heidary, V. Maisonneuve, A. Guiet, N. Kornienko | Chemical Science - issue 40, 9209-9218 - Sep 2019
- [37] "Operando Raman probing of electrocatalytic biomass oxidation on gold nanoparticle surfaces"59 N. Heidary, N. Kornienko | Chemical Commununications - issue 80, 11996-11999 - Sep 2019
- [36] "Probing CO2 conversion chemistry on nanostructured surfaces with operando vibrational spectroscopy"60 N. Heidary, K. H. Ly, N. Kornienko | Nano Letters - vol 19, issue 8, 4817-4826 - Aug 2019
- [35] "Advancing Techniques for Investigating the Enzyme-Electrode Interface"61 N. Kornienko, K. H. Ly, W. E. Robinson, N. Heidary, J. Z. Zhang, E. Reisner | Accounts of Chemical Research - vol 52, issue 5, 1439-1448 - May 2019
- [34] "Bio-inspired synthesis of reduced graphene oxide-wrapped Geobacter sulfurreducens as a hybrid electrocatalyst for efficient oxygen evolution reaction"62 S. Kalathil, K. Katuri, A. Alzami, P. Pedireddy, N. Kornienko, P. Costa, P. Saikally | Chemistry of Materials - vol 31, issue 10, 3686-3693 - May 2019
- [33] "Interfacing formate dehydrogenase with metal oxides for the reversible electrocatalysis or solar-driven reduction of carbon dioxide"63 M. Miller, W. E. Robinson, A. R. Oliveira, N. Heidary, N. Kornienko, J. Warnan, I. A. C. Pereira, E. Reisner | Angewandte Chemie Int. Edition - vol 58, issue 14, 4601-4605 - Mar 2019
- [32] "Artificial Photosynthesis with Metal and Covalent Organic Frameworks (MOFs and COFs): Challenges and Prospects in Fuel-Forming Electrocatalysis"64 N. Heidary, T. G.A.A Harris. K.H. Ly, N. Kornienko | Physiologia Plantarum - vol 166, issue 1, 460-471 - May 2019
- [31] "Oxygenic Photoreactivity in Photosystem II Studied by Rotating Ring Disk Electrochemistry"65 N. Kornienko, J. Z. Zhang, K. Ly, K. P. Sokol, A. Fantuzzi, R. van Grondelle, A. W. Rutherford, E. Reisner | JACS - vol 140, issue 51, 17923–17931 - Okt 2018
- [30] "Bias-free photoelectrochemical water splitting with photosystem II on a dye-sensitised photoanode wired to hydrogenase"66 K. P. Sokol, W. E. Robinson, J. Warnan, N. Kornienko, J. Zhang, A. Ruff. E. Reisner | Nature Energy - vol 3, issue 11, 944–951 - Nov 2018
- [29*] "Interfacing nature’s catalytic machinery with synthetic materials for semi-artificial photosynthesis"67 Nikolay Kornienko, Jenny Zhang, Kelsey K. Sakimoto, Peidong Yang, Erwin Reisner | Nature Nanotechnology 13, 890–899 - 2018
- [28] "Catalysis by design: development of a bifunctional water splitting catalyst through an operando measurement directed optimization cycle"68 Nikolay Kornienko, Nina Heidary, Giannantonio Cibin, Erwin Reisner | Chemical Science - issue 9, 5322-5333 - May 2018
- [27] "Aerobic conditions enhance the photocatalytic stability of CdS/CdOx quantum dots"69 David Wakerley, Khoa Ly, Nikolay Kornienko, Katherine Orchard, Moritz Kuehnel, Erwin Reisner | Chemistry: A European Journal - vol 24, issue 69, 18385-18388 - Dec 2018
- [26] "Solar Water Splitting with a Hydrogenase Integrated in Photoelectrochemical Tandem Cells"70 Dong Heon Nam, Jenny Z. Zhang, Virgil Andrei, Nikolay Kornienko, Nina Heidary, Andreas Wagner, Kenichi Nakanishi, Katarzyna P. Sokol, Barnaby Slater, Ingo Zebger, Stephan Hofmann, Juan C. Fontecilla-Camps, Chan Beum Park , Erwin Reisner | Angewandte Chemie - vol 57, issue 33, 10595-10599 - Aug 2018
- [25] "Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts"71 Hyo Won Kim, Michael B Ross, Nikolay Kornienko, Liang Zhang, Jinghua Guo, Peidong Yang, Bryan D McCloskey | Nature Catalysis - vol 1, issue 4, 282-290 - Apr 2018
- [24] "Enhancing Catalysis through Substitute-Driven Redox Tuning"72 Nikolay Kornienko | Joule - vol 2, issue 2, 207-209 - Feb 2018
- [23] "Physical Biology of the Materials–Microorganism Interface"73 Kelsey K Sakimoto, Nikolay Kornienko, Stefano Cestellos-Blanco, Jongwoo Lim, Chong Liu, Peidong Yang | JACS - vol 140, issue 6, 1978-1985 - Feb 2018
- [22] "Extending the Compositional Space of Mixed Lead Halide Perovskites by Cs, Rb, K, and Na Doping"74 T. J Jacobsson , S. Svanström, V. Andrei, J. P. H. Rivett, N. Kornienko, B. Philippe, U. B. Cappel, H. Rensmo, F. Deschler, and G. Boschloo | Journal of Physical Chememistry C, - vol 122, issue 25, 13548-13557 - Jun 2018
- [21] "Reticular Electronic Tuning of Porphyrin Active Sites in Covalent Organic Frameworks for Electrocatalytic Carbon Dioxide Reduction"75 C. Dierks, S. Lin, N. Kornienko, E. Kapustin, E. Nichols, C. Zhu, Y. Zhao, C. Chang, and O. M. Yaghi | JACS - vol 140, issue 3, 1116-1122 - Jan 2018
- [20] "Critical Role of Methylammonium Librational Motion in Methylammonium Lead Iodide (CH3NH3PbI3) Perovskite Photochemistry"76 M. Park, N. Kornienko, S. E. Reyes-Lillo, M. Lai, J. B. Neaton, P. Yang, and R. A. Mathies | Nano Letters - vol 17, issue 7, 4151–4157 - Jul 2017
- [19] "Cyborgian Material Design for Solar Fuel Production: The Emerging Photosynthetic Biohybrid Systems"77 K. Sakimoto, N. Kornienko, P. Yang | Accounts of Chemical Research - vol 50, issue 3, 476-481 - Mar 2017
- [18] "Spectroscopic elucidation of energy transfer in hybrid inorganic–biological organisms for solar-to-chemical production"78 N. Kornienko, K. Sakimoto, D. Herlihy, S. Nguyen, A. P. Alivisatos, C. B. Harris, A. Schwartzberg, P. Yang Proc | PNAS - vol 113, issue 42, 11750-11755 - Oct 2016
- [17] "Atomic Resolution Imaging of Halide Perovskites"79 Y. Yu, D. Zhang, C. Kisielowski, L. Dou, N. Kornienko, Y. Bekenstein, A. P. Alivisatos, P. Yang | Nano Letters - vol 16, issue 12, 7530-7535 - Dec 2016
- [16] "Synthesis and Composition Tunable and Highly Luminescent Cesium Lead Halide Nanowires through Anion-Exchange Reactions"80 D. Zhang, Y. Yang, Y. Yu, N. Gibson, A. Wong, S. Eaton, N. Kornienko, Q. Kong, M. Lai, Y. Bekenstein, A. P. Alivisatos, S. R. Leone, P. Yang | JACS - vol 138, issue 23, 7236-7239 - Jun 2016
- [15] "Anisotropic Phase Segregation and Migration of Pt in Nanocrystals En Route to Nanoframe Catalysts"81 Z. Niu, B. Becknell, Y. Yu, D. Kim, C. Chen, N. Kornienko, G. Somorjai, P. Yang | Nature Materials - vol 15, issue 11, 1188-1194 - Nov 2016
- [14] "Growth and Photoelectrochemical Energy Conversion of Wurtzite Indium Phosphide Nanowire Arrays"82 N. Kornienko, N. Gibson, H. Zhang, S. W. Eaton, S. Aloni, S. Leone, P. Yang | ACS Nano - vol 10, issue 5, 5525-5535 - May 2016
- [13] "Single Nanowire Photoelectrochemistry"83 Y. Su, C. Liu, S. Brittman, J. Tang, A. Fu, N. Kornienko, Q. Kong, P. Yang | Nature Nanotechnology - vol 11, issue 7, 609-612 - Jul 2016
- [12] "TiO2/BiVO4 Heterostructure Photoanodes Based on Type II Band Allignment"84 J. Resarco, H. Zhang, N. Kornienko, N. Becknell, H. Lee, J. Guo, A. Briseno, P. Yang | ACS Central Science - vol 2, issue 2, 80-88 - Feb 2016
- [11] "Low-Temperature Solution-Phase Growth of Silicon and Silicon-Containing Alloys"85 J. Sun, F. Cui, C. Kiseilowski, Y. Yu, N. Kornienko, P. Yang | The Journal of Physical Chemistry C - vol 120, issue 37 - 20525-20529 - Sep 2016
- [10] "Atomic Level Structure of Pt3Ni Nanoframe Electrocatalysts by In-Situ X-Ray Absorption Spectroscopy"86 N. Becknell, Y. Kang, C. Chen, J. Resasco, N. Kornienko, J. Guo, N. Markovic, G. Somorjai, V. Stamenkovic, P. Yang | JACS - vol 137, issue 50, 15817-15824 - Dez 2015
- [9] "Atomically Thin Two-Dimensional Organic-Inorganic Hybrid Perovskites"87 L. Duo, A. Wong, Y. Yu, M. Lai, N. Kornienko, S. Eaton, A. Fu, C. Bishak, J. Ma, T. Ding, N. Ginsberg, L. Wang, A. Alivisatos, P. Yang | Science - vol 349, no 6255, 1518-1521 - Sep 2015
- [8] "Metal-Organic Frameworks for Electrocatalytic Reduction of Carbon Dioxide"88 N. Kornienko*, Y. Zhao, C. Kley, C. Zhu, D. Kim, S. Lin, C. Chang, O. Yaghi, P. Yang | JACS - vol 137, issue 44, 14129-14135 - Nov 2015
- [7] "Covalent Organic Frameworks Comprising Cobalt Porphyrins for Catalytic CO2 Reduction in Water"89 S. Lin*, C. Dierks, Y. Zhang*, N. Kornienko, E. Nichols, Y. Zhao, A. Paris, D. Kim, P. Yang, O. Yaghi, C. Chang | Science - vol 349, issue 6253, 1208-1213 - Aug 2015
- [6] "Operando Spectroscopic Analysis of an Amorphous Cobalt Sulfide Hydrogen Evolution Electrocatalyst"90 N. Kornienko, J. Resasco, N. Becknell, C. Jiang, Y. Liu, K. Nie, X. Sun, J. Guo, S. Leone, P. Yang | JACS - vol 137, issue 23, 7448-7455 - Jun 2015
- [5] "Solution Phase Synthesis of Indium Gallium Phosphide Alloy Nanowires"91 N. Kornienko, D. Whitmore, Y. Yu, S. Leone and P. Yang | ACS Nano - vol 9, issue 4, 3951-3960 - Apr 2015
- [4] "Mesoscopic Constructs of Ordered and Oriented Metal-Organic Frameworks on Plasmonic Silver Nanocrystals"92 Y. Zhao*, N. Kornienko, Z. Liu,C. Zhu, S. Asahina, T. Kuo, W. Bao, C. Xie, O. Terasaki, P. Yang, O. Yaghi | JACS - vol 137, issue 6, 2199-2202 - Feb 2015
- [3] "Visible-Light Photoredox Catalysis: Selective Reduction of Carbon Dioxide to Carbon Monoxide by a Nickel N-Heterocyclic Carbene- Isoquinoline Complex"93 V Thoi, N. Kornienko, C Margarit, P. Yang and C. Chang | JACS - vol 135, issue 38, 14413-14424 - Sep 2013
- [2] "Reflectivity Enhanced Two-Dimensional Dielectric Particle Array Monolayer Diffraction"94 A. Tikhonov, N. Kornienko, J. Zhang, L. Wang and S.A. Asher | JNP - vol 6, issue 1 - Jun 2012
- [1] "2-D Array Photonic Crystal Sensing Motif"95 J. Zhang, L. Wang, J. Luo, A. Tikhonov, N. Kornienko, and S.A. Asher | J. Am. Chem. Soc. - vol 133, issue 24, 9152-9155 - Jun 2011
* ausgewählte aktuelle Publikationen
Ausgewählte aktuelle Publikationen
[92] Accelerating lithium-mediated nitrogen reduction through an integrated palladium membrane hydrogenation reactor
Lithium-mediated N2 reduction reaction (LiNRR) is regarded as the most robust route towards electrifying NH3 synthesis. However, in this reaction geometry, hydrogen atoms typically supplied through electrolyte degradation, via H2 oxidation or a combination of both, hampering the efficiency of the process. In this work we provide an alternative H-source by merging a Pd Membrane reactor (PMR) with a LiNRR reactor in a unique dual-reactor setup. Specifically, use a Pd membrane that extracts H atoms directly from H2O and transfers them across the membrane to an electrodeposited Li layer operating under non-aqueous LiNRR conditions. We show that these H2O-derived H-atoms are used directly to synthesize NH3 in the presence of N2 and electrodeposited Li, thereby opening orthogonal reaction pathways within the metal-mediated nitrogen reduction concept.
[90] Dynamic active sites behind Cu-based electrocatalysts: Original or restructuring-induced catalytic activity
Structural dynamics in electrocatalysts under operating conditions, including restructuring, dissolution/redeposition, and single-site-to-cluster transitions, are commonly observed phenomena but are often poorly understood. Cu-based catalysts, entailing single-atom catalysts (SACs), molecular catalysts, and nanostructured catalysts, have shown promise in key electrochemical reactions such as CO2 reduction, NO3− reduction, and C–N coupling and are particularly prone to structural changes as they carry out these reactions. While the fully accurate prediction of restructuring-induced activity remains an ongoing challenge, this review offers a comprehensive analysis of Cu-based catalysts, focusing on the dynamic behavior of Cu active sites, which can undergo structural changes during electrocatalytic reactions, thereby impacting catalytic performance and selectivity. Taking insights from advanced in situ/operando techniques, such as X-ray absorption spectroscopy (XAS), this study identifies and evaluates a set of Cu-based electrocatalysts under electrocatalytic reduction reactions and distinguishes the dynamic active sites between original and restructuring-induced forms.
8Shuai Chen, Farzaneh Farzinpour, N. Kornienko
Chem - online - May 20256
[88] Best practices for in-situ and operando techniques within electrocatalytic systems
In-situ and operando techniques in heterogeneous electrocatalysis are a powerful tool used to elucidate reaction mechanisms. Ultimately, they are key in determining concrete links between a catalyst’s physical/electronic structure and its activity en route to designing next-generation systems. To this end, the exact execution and interpretation of these lines of experiments is critical as this determines the strength of conclusions that can be drawn and what uncertainties remain. Instead of focusing on how techniques were used to understand systems, as is the case with most reviews on the topic, this work instead initiates a nuanced discussion of 1) how to best carry out each technique and 2) initiate a nuanced analysis of which level of insights can be drawn from the set of in-situ or operando experiments/controls carried out. We focus on several commonly used techniques, including vibrational (IR, Raman) spectroscopy, X-ray absorption spectroscopy and electrochemical mass spectrometry. In addition to this, we include sections of reactor design and the link with theoretical modelling that are applicable across all techniques. While we focus on heterogeneous electrocatalysis, we make links when appropriate to the areas of photo- and thermo-catalytic systems. We highlight common pitfalls in the field, how to avoid them, and what sets of complementary experiments may be used to strengthen the analysis. We end with an overview of what gaps remain in in-situ and operando techniques and what innovations must be made to overcome them.
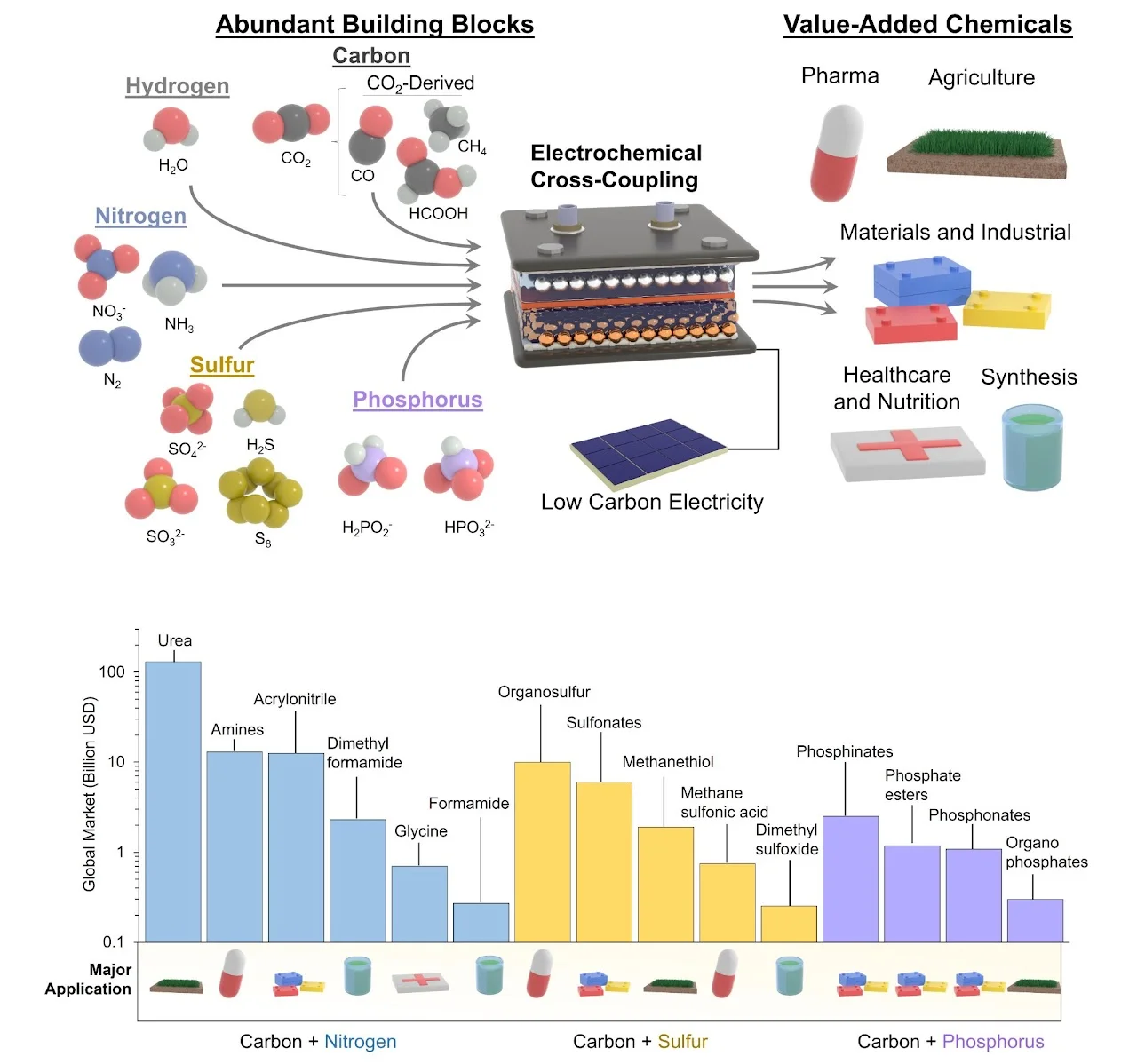
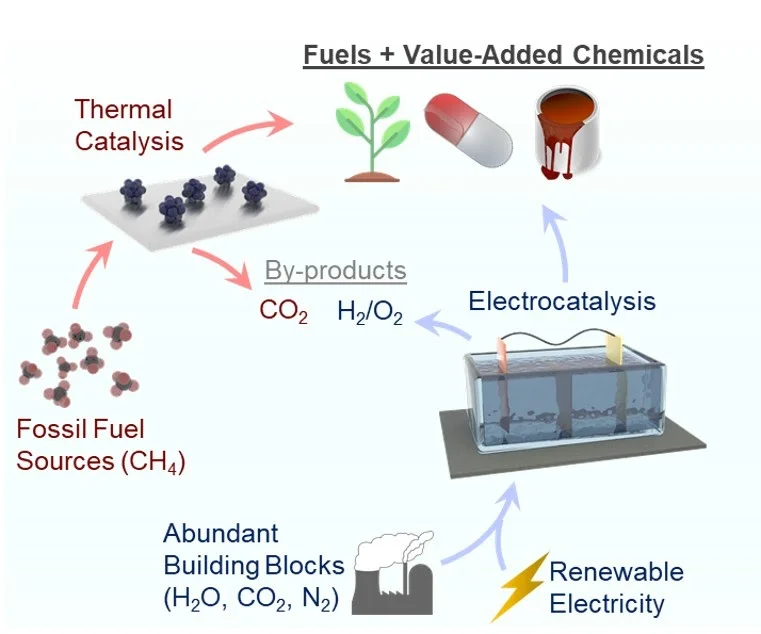
[76] Heterogeneous electrosynthesis of C–N, C–S and C–P products using CO2 as a building block
Electrochemical CO2 reduction (CO2R) has garnered interest as a sustainable route for the production of carbon-based fuels. Against this backdrop, this perspective explores how the scope and consequent impact of CO2R can be expanded through coupling with heteroatomic co-reactants. We begin with an evaluation of societal demand for basic C-X (C-N, C-S and C-P) bond containing chemicals and a look into how they are currently synthesized. Established routes for heteroatom coupling are then contrasted with emerging electrosynthetic approaches that use CO2 as a building block, which we classify into three distinct categories. Within each identified class of electrosynthetic coupling, a critical examination pinpoints the key aspects behind the catalyst, reactor, and molecule-specific reactivity that enables the coupling pathway. The perspective is concluded with a forward-looking analysis of what catalytic chemistry needs to be developed in the context of sustainable electrosynthesis and how computational tools may accelerate the progress in a joint effort. We further discuss upcoming challenges in both system design and technoeconomic/life cycle analysis that need to be addressed as this technology matures implementation at scale.
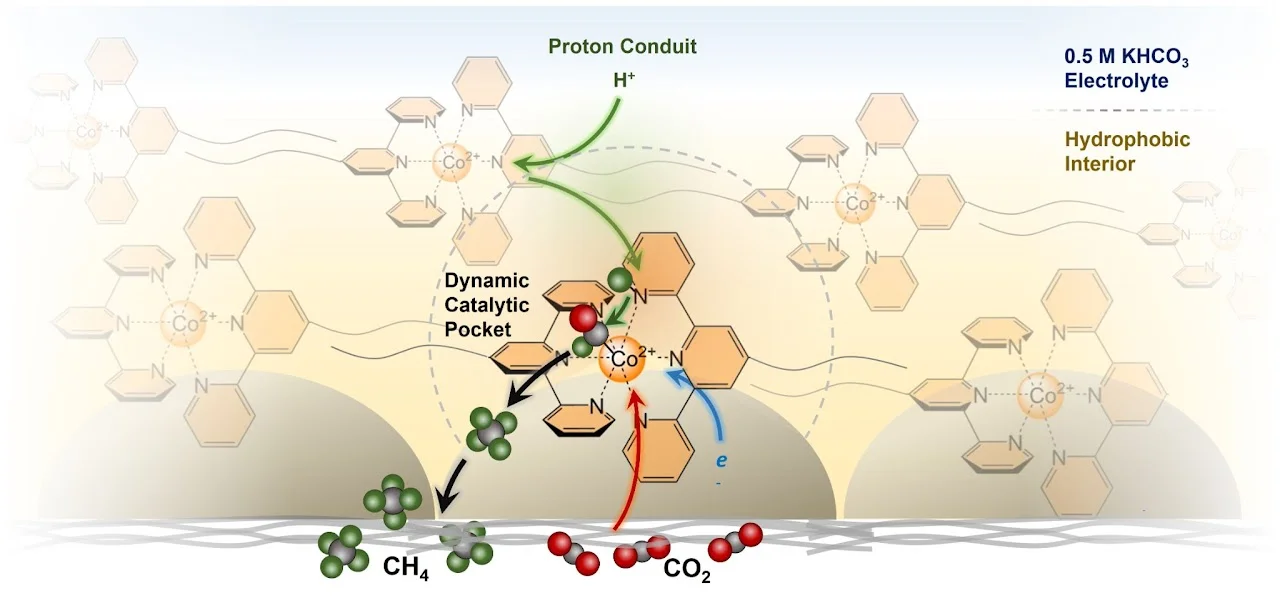
[73] Hydrophobic assembly of molecular catalysts at the gas–liquid–solid interface drives highly selective CO2 electromethanation
The modularity of molecular catalysts enables the tuning of both active site and peripheral units to maximize functionality, thus rendering them as ideal model systems to explore fundamental concepts in catalysis. Hydrophobicity is often regarded as an undesirable aspect that hinders their dissolution in aqueous electrolytes. In contrast, we modified established cobalt terpyridine catalysts with hydrophobic perfluorinated alkyl side chains and took advantage of their hydrophobic character by utilizing them not as dissolved species in an aqueous electrolyte but at the gas-liquid-solid interfaces on a gas diffusion electrode (GDE) applied towards the electrochemical reduction of CO2. We found that the self-assembly of these perfluorinated units on the GDE surface results in a catalytic system selective for CH4 production, whereas every other Co terpyridine catalyst reported before was only selective for CO or formate. An array of mechanistic and operando spectroscopic investigations suggests a mechanism in which the pyridine units function as proton shuttles that deliver protons to the dynamic hydrophobic pocket in which CO2 reduction takes place. Finally, optimizing the system by integrating fluorinated carbon nanotubes as a hydrophobic conductive scaffold leads to a Faradaic efficiency for CH4 production above 80% at rates above 10 mA/cm-2, thus far unprecedented for a molecular electrocatalytic system.
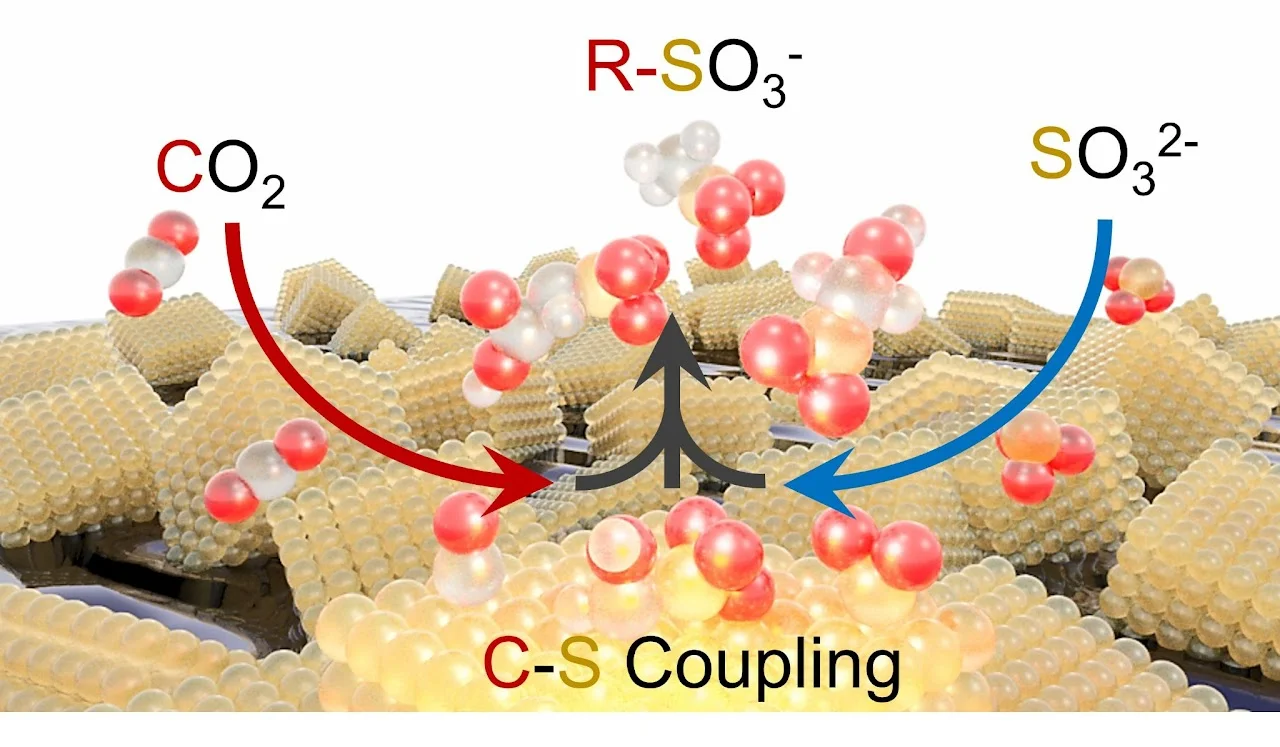
[69] Electrochemical Formation of C-S Bonds from CO2 and Small Molecule Sulfur Species
The formation of C-S bonds is an important step in the synthesis of pharmaceutical, biological, and chemical products. A very attractive green route to C-S bond containing species would be one driven through electrocatalysis using abundant small molecule precursors but examples within this context are largely absent from the literature. To this end, this work demonstrates the use of CO2 and SO32- as cheap building blocks that couple on the surface Cu-based heterogeneous catalysts to form hydroxymethanesulfonate, sulfoacetate and methane sulfonate for the first time, with Faradaic efficiencies of up to 9.5%. A combination of operando measurements and computational modelling reveal that *CHOH formed on metallic Cu is a key electrophilic intermediate that is nucleophilically attacked by SO32- in the principal C-S bond forming step. In all, the proof-of-concept for electrocatalytic C-S bond formation and mechanistic insights gained stand to substantially broaden the scope of the emerging field of electrosynthesis.
[68] Construction of C–N bonds from small-molecule precursors through heterogeneous electrocatalysis
Energy-intensive thermochemical processes within chemical manufacturing are a major contributor to global CO2 emissions. With the increasing push for sustainability, the scientific community is striving to develop renewable energy-powered electrochemical technologies in lieu of CO2-emitting fossil-fuel-driven methods. However, to fully electrify chemical manufacturing, it is imperative to expand the scope of electrosynthetic technologies, particularly through the innovation of reactions involving nitrogen-based reactants. This Review focuses on a rapidly emerging area, namely the formation of C–N bonds through heterogeneous electrocatalysis. The C–N bond motif is found in many fertilizers (such as urea) as well as commodity and fine chemicals (with functional groups such as amines and amides). The ability to generate C–N bonds from reactants such as CO2, NO3– or N2 would provide sustainable alternatives to the thermochemical routes used at present. We start by examining thermochemical, enzymatic and molecular catalytic systems for C–N bond formation, identifying how concepts from these can be translated to heterogeneous electrocatalysis. Next, we discuss successful heterogeneous electrocatalytic systems and highlight promising research directions. Finally, we discuss the remaining questions and knowledge gaps and thus set the trajectory for future advances in heterogeneous electrocatalytic formation of C–N bonds.
[47] Electrochemically Triggered Dynamics Within a Hybrid Metal-Organic Electrocatalyst
A wide array of systems, ranging from enzymes to synthetic catalysts, exert adaptive motifs to maximize their functionality. In a related manner, select metal-organic frameworks (MOFs) and related systems exhibit structural modulations under stimuli such as the infiltration of guest species. Probing their responsive behavior in-situ is a challenging but important step towards understanding their function and subsequently building from there. In this report, we investigate the dynamic behavior of an electrocatalytic Mn-porphyrin containing MOF system (Mn-MOF). We discover, using a combination of electrochemistry and in-situ probes of UV-Vis absorption, resonance Raman and infrared spectroscopy, a restructuration of this system via a reversible cleavage of the porphyrin carboxylate ligands under an applied voltage. We further show, by combining experimental data and DFT calculations, as a proof of concept, the capacity to utilize the Mn-MOF for electrochemical CO2 fixation and to spectroscopically capture the reaction intermediates in its catalytic cycle. The findings of this work and methodology developed opens opportunities in the application of MOFs as dynamic, enzyme-inspired electrocatalytic systems.
[29] Interfacing nature’s catalytic machinery with synthetic materials for semi-artificial photosynthesis
Semi-artificial photosynthetic systems aim to overcome the limitations of natural and artificial photosynthesis while providing an opportunity to investigate their respective functionality. The progress and studies of these hybrid systems is the focus of this forward-looking perspective. In this Review, we discuss how enzymes have been interfaced with synthetic materials and employed for semi-artificial fuel production. In parallel, we examine how more complex living cellular systems can be recruited for in vivo fuel and chemical production in an approach where inorganic nanostructures are hybridized with photosynthetic and non-photosynthetic microorganisms. Side-by-side comparisons reveal strengths and limitations of enzyme- and microorganism-based hybrid systems, and how lessons extracted from studying enzyme hybrids can be applied to investigations of microorganism-hybrid devices. We conclude by putting semi-artificial photosynthesis in the context of its own ambitions and discuss how it can help address the grand challenges facing artificial systems for the efficient generation of solar fuels and chemicals.
Links
- https://doi.org/10.1021/acs.energyfuels.5c02150
- https://doi.org/10.1002/cssc.202501180
- https://doi.org/10.1021/acs.inorgchem.5c02152
- https://doi.org/10.1038/s41467-025-62088-z
- https://doi.org/10.1093/nsr/nwaf198
- https://doi.org/10.1016/j.chempr.2025.102575
- https://doi.org/10.5802/crchim.371
- https://doi.org/10.1038/s41467-025-57563-6
- https://doi.org/10.1016/j.cclet.2025.110988
- https://doi.org/10.1021/acsami.4c13187
- https://doi.org/10.1002/advs.202414431
- https://doi.org/10.1038/s41929-024-01217-1
- https://doi.org/10.1021/acsami.4c09575
- https://doi.org/10.1021/acscatal.4c01448
- https://doi.org/10.1039/D4SU00134F
- https://doi.org/10.1021/acsaem.4c00259
- https://doi.org/10.1016/j.apcatb.2024.124061
- https://doi.org/10.1016/j.checat.2024.100948
- https://doi.org/10.1016/j.isci.2024.109482
- https://doi.org/10.1038/s44160-024-00530-8
- https://doi.org/10.1039/D3SC05362H
- https://doi.org/10.1021/acscatal.3c01673
- https://doi.org/10.1038/s41557-024-01650-6
- https://doi.org/10.26434/chemrxiv-2022-07rlk
- https://doi.org/10.1038/s41929-022-00860-w
- https://doi.org/10.1016/j.checat.2023.100595
- https://doi.org/10.1038/s44160-023-00303-9
- https://doi.org/10.1038/s41570-022-00379-5
- https://doi.org/10.1063/5.0090147
- https://doi.org/10.1016/j.joule.2021.12.014
- https://doi.org/10.1016/j.xcrp.2021.100682
- https://doi.org/10.1002/celc.202101632
- https://doi.org/10.1016/j.checat.2021.10.016
- https://doi.org/10.1016/j.checat.2022.03.002
- https://doi.org/10.1016/j.chempr.2021.09.003
- https://www.cell.com/iscience/fulltext/S2589-0042%2821%2901017-8#relatedArticles
- https://doi.org/10.1016/j.checat.2022.02.005
- https://doi.org/10.1002/cssc.202101587
- https://doi.org/10.1039/D1SC06590D
- https://doi.org/10.1039/D1CC01429C
- https://doi.org/10.1039/D1CC01155C
- https://doi.org/10.1039/D1SC00573A
- https://doi.org/10.1021/acsaem.0c02417
- https://doi.org/10.1039/D0NR07508F
- https://doi.org/10.1039/D0NJ03526B
- https://doi.org/10.1016/j.nanoen.2020.105311
- https://doi.org/10.1021/acs.nanolett.0c02452
- https://doi.org/10.1039/D0CC02815K
- https://pubs.acs.org/doi/10.1021/jacs.0c04758
- https://doi.org/10.1039/D0CC03084H
- https://doi.org/10.1002/adfm.202002633
- https://doi.org/10.26434/chemrxiv.12014910.v1
- https://doi.org/10.1021/jacs.9b13077
- https://doi.org/10.1039/C9NJ05892C
- https://doi.org/10.1039/D0SC00136H
- https://doi.org/10.1021/acscatal.9b04221
- https://doi.org/10.1016/j.cclet.2019.11.001
- https://doi.org/10.1039/C9SC04027G
- https://doi.org/10.1039/C9CC06646B
- https://doi.org/10.1021/acs.nanolett.9b01582
- https://doi.org/10.1021/acs.accounts.9b00087
- https://doi.org/10.1021/acs.chemmater.9b00394
- https://doi.org/10.1002/anie.201814419
- https://doi.org/10.1111/ppl.12935
- https://doi.org/10.1021/jacs.8b08784
- https://doi.org/10.1038/s41560-018-0232-y
- https://www.nature.com/articles/s41565-018-0251-7
- https://doi.org/10.1039/C8SC01415A
- https://doi.org/10.1002/chem.201802353
- https://doi.org/10.1002/anie.201805027
- https://doi.org/10.1038/s41929-018-0044-2
- https://doi.org/10.1016/j.joule.2018.01.005
- https://doi.org/10.1021/jacs.7b11135
- https://doi.org/10.1021/acs.jpcc.7b12464
- https://doi.org/10.1021/jacs.7b11940
- https://doi.org/10.1021/acs.nanolett.7b00919
- https://doi.org/10.1021/acs.accounts.6b00483
- https://doi.org/10.1073/pnas.1610554113
- https://doi.org/10.1021/acs.nanolett.6b03331
- https://doi.org/10.1021/jacs.6b03134
- https://doi.org/10.1038/nmat4724
- https://doi.org/10.1021/acsnano.6b02083
- https://doi.org/10.1038/nnano.2016.30
- https://doi.org/10.1021/acscentsci.5b00402
- https://doi.org/10.1021/acs.jpcc.5b08289
- https://doi.org/10.1021/jacs.5b09639
- https://doi.org/10.1126/science.aac7660
- https://doi.org/10.1021/jacs.5b08212
- https://doi.org/10.1126/science.aac8343
- https://doi.org/10.1021/jacs.5b03545
- https://doi.org/10.1021/nn507335j
- https://doi.org/10.1021/ja512951e
- https://doi.org/10.1021/ja4074003
- https://doi.org/10.1117/1.JNP.6.063509
- https://doi.org/10.1021/ja201015c
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/91-2025-selected.jpg
- https://www.uni-bonn.de/en/news/141-2025
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/89-2025-selected.jpg
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/87-2025-selected.jpg
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/76-2024.jpg
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/73-2024.jpg
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/69-2023.jpg
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/copy_of_68-2022.jpg
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/47-2020.png
- https://doi.org/10.1021/jacs.0c04758
- https://www.chemie.uni-bonn.de/kornienko/media-folder/media-publications/29-2018.png
- https://doi.org/10.1038/s41565-018-0251-7
- https://www.chemie.uni-bonn.de/kornienko/de/publications