Ausgewählte Publikationen
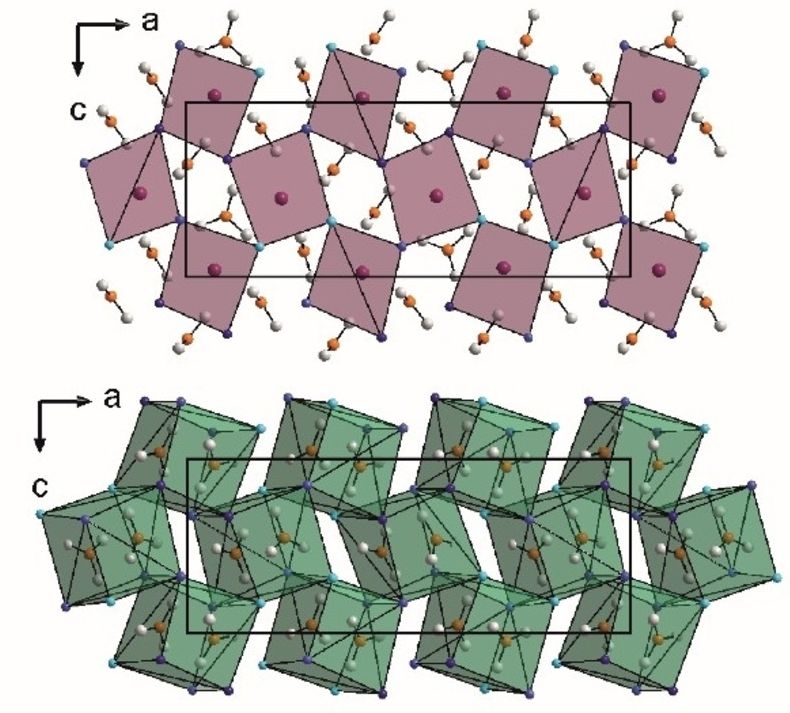
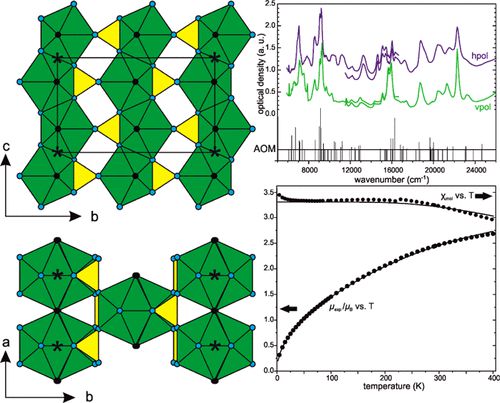
Anti-polar 2D-metallicity with tuneable valence Wx+ (5 ≤ x ≤ 5.6) in the layered monophosphate tungsten bronzes [Ba(PO4)2]WmO3m–3
The newly discovered series of layered monophosphate tungsten bronzes (L-MPTB) [Ba(PO)2]WmO3m–3 consist of m-layer-thick slabs of WO6 octahedra separated by barium-phosphate spacers. They display a 2D metallic behavior confined in the central part of the perovskite slabs. Here, we report the missing m = 2 member of this series, containing the rather uncommon W5+ oxidation state. We have analyzed its structure-property relationships in relation to the other members of the L-MPTB family. In particular, we have determined its crystal structure by means of single-crystal X-ray and electron diffraction and investigated its physical properties from resistivity, Seebeck-coefficient and heat-capacity measurements combined with first-principles calculations. All the L-MPTB compounds show metallic behavior down to 1.8 K without any clear charge-density-wave (CDW) order. The m = 2 member, however, displays an increased influence of the spacer that translates into anisotropic negative thermal expansion, reversed thermopower and reversed crystal-field splitting of the tungsten t2g orbitals. Our analysis of the full [Ba(PO4)2]WmO3m–3 series reveals a systematic and significant W off-centering in their octahedral coordination. We identify the resulting anti-polar character of these W displacements as the crucial aspect behind the 2D metallicity of these systems: It leads to the presence of bound charges whose screening determines the distribution of mobile charges, tending to accumulate at the center of the [WmO3–m] block. We argue that this mechanism is analogous to enhanced conductivity observed for charged domain walls in ferroelectrics, thus providing a general design rule to promote 2D metallicity in layered systems.
Hicham Nimoh, Angel M. Arevalo-López, Quintin N. Meier, Claire Minaud, Marielle Huvé, Frédéric Capet, Andrés Cano, Robert Glaum* and Olivier Mentré*
J. Am. Chem. Soc. (2024) online.


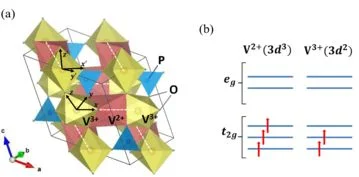
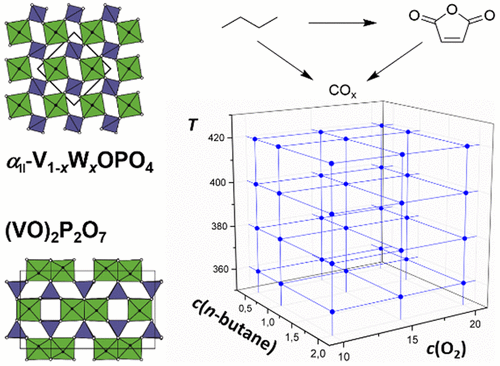
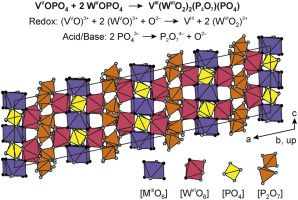
Niobium-insertion into αII-VOPO4: Tuning catalytic properties for selective oxidation
A holistic understanding of the key catalytic features of vanadyl(IV) pyrophosphate enabling high maleic anhydride (MAN) yields in n-butane oxidation has fostered a debate which has continued since the finding of the catalyst. Under reaction conditions, vanadium(V) orthophosphate structure fragments were detected on the surface of the catalyst. However, single-phase αII- and β-VVOPO4 reveal a much lower catalytic performance. This study shows that introducing Nb into αII-VOPO4 forming a solid solution (V1-xNbx)OPO4 yields a bulk material with tunable catalytic properties. Selectivities of SMAN = 48% at a conversion of Xn‑butane = 30% on (V0.1Nb0.9)OPO4 are shown to be related to the isolation of surface V-sites, which surpass known VOPO4 catalysts by far. A boost in the overall n-butane consumption and MAN selectivity under alkane-rich feed conditions is shown to be another characteristic of (V1-xNbx)OPO4, leading to a highly increased MAN productivity. XPS studies reveal that a progressive replacement of V by Nb induces a reduction of the averaged oxidation state of near-surface V from +4.7 to +4.3, a finding that correlates linearly with an elevated MAN selectivity. This study experimentally confirms site isolation and electronic environment of the near-surface V-species as the key catalytic properties, from which catalyst design rules are derived to optimize partial oxidation reactions.
Frederik Rüther, Rhea Machado, Esteban Gioria, Sylvia L. Kunz, Knut Wittich, Patricia Löser, Michael Geske,* Stephan A. Schunk, Robert Glaum, Frank Rosowski
ACS Catalysis 13 (2023) 3295-3307.
The first triel oxonitridoborates AlB4O6N, Al0.97Cr0.03B4O6N, and Al0.83Cr0.17B4O6N being in competition to ruby
AlB4O6N, Al0.97Cr0.03B4O6N, and Al0.83Cr0.17B4O6N are the first
representatives of the recently established structure-family of
oxonitridoborates containing Al3+. These compounds are isotypic to CrB4O6N
and are synthesized in a multi-anvil press under
high-pressure/high-temperature conditions of 7.0 GPa/1350 °C. Structural
refinement by single-crystal X-ray diffraction shows that they crystallize in the
space group P63mc (no. 186) with two formula units per cell. Detailed
characterization including high-temperature X-ray powder diffraction
(HT-XRD), electron probe microanalysis (EPMA), measurements of second
harmonic generation (SHG), hardness, photoluminescence properties,
vibrational spectroscopy, and band structure calculations reveal intriguing
physicochemical properties that strongly resemble the famous material ruby.
Ingo Widmann, Gülsüm Kinik, Maximilian Jähnig, Robert Glaum, Marcus Schwarz,
Christina Wüstefeld, Dirk Johrendt, Martina Tribus, Clivia Hejny, Lkhamsuren Bayarjargal,
Leonid Dubrovinsky, Gunter Heymann, Markus Suta,* and Hubert Huppertz*
Angew. Chem. Int. Ed. (2024) 63.


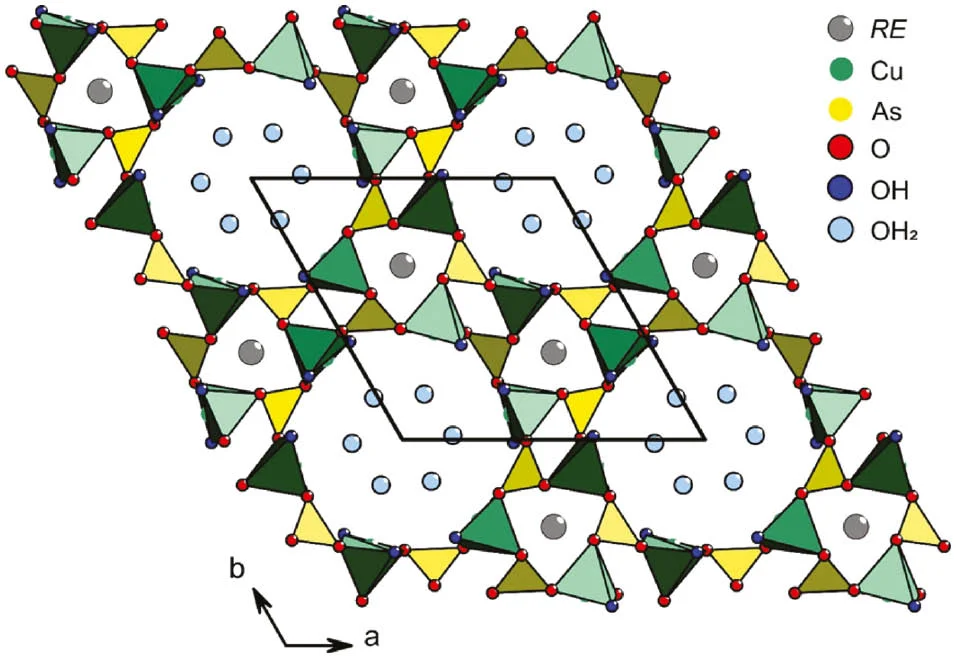
Lithium Copper(I) Orthophosphates Li3–xCuxPO4: Synthesis, Crystal Structures, and Electrochemical Properties
Along the quasi-binary section Li3PO4 - CuI3PO4 three different phases Li3–xCuIxPO4 each with extended homogeneity range occur under equilibrium conditions (650≤ϑ≤700 °C). According to single-crystal X-ray structure analyses Phase 1 (0<x≤0.7) adopts the HT- or β-Li3PO4 structure type [Li2.6CuI0.4PO4, Pnma (no. 62), Z=4, a=10.4612(2) Å, b=6.1690(3) Å, c=4.9854(2) Å, R1=0.023, wR2=0.062, Goof=1.12] and Phase 2 (0.9≤x≤1.8) is isotypic to LT- or α-Li3PO4 [Li2.05CuI0.95PO4, Pnm21 (no. 31), Z=2, a=6.2113(8) Å, b=5.2597(7) Å, c=4.9904(5) Å, R1=0.040, wR2=0.108, Goof=0.98]. A preliminary structure model for the copper-rich Phase 3 (2.1≤x≤2.8) ["Li0.6CuI2.4PO4", P3 (no. 147), a=6.223(1) Å, c=5.3629(5) Å] could be refined to R1=0.07. Sharp 31P-MAS-NMR resonances observed in the spectra of Li2.6CuI0.4PO4 (δiso=10.4 ppm), Li2.05CuI0.95PO4 (δiso=12.4 ppm), and Li0.84CuI2.16PO4 (δiso=10.9 ppm) provide evidence for the absence of paramagnetic Cu2+ ions. Pure copper(I) orthophosphate CuI3(PO4) exists as a homogeneous melt (≥800 °C) and can be obtained as thermodynamically metastable solid by quenching. It is isotypic to Phase 3 [a=6.284(3) Å, c=5.408(5) Å]. Electrochemical delithiation of Li2.05CuI0.95PO4 (C/10, C/30) indicates two partially reversible oxidation processes between 3.75 V and 4.80 V (vs. Li0/Li+).
Katharina Snyder, Branimir Raguž, Wilfried Hoffbauer, Robert Glaum, Hartmut Ehrenberg, Markus Herklotz
Z. Anorg. Allg. Chem. 2014, 640, (5), 944-951
Neueste Publikationen

[186]
"Anti-polar 2D-metallicity with tuneable valence Wx+ (5 ≤ x ≤ 5.6) in the layered monophosphate tungsten bronzes [Ba(PO4)2]WmO3m–3"
Hicham Nimoh, Angel M. Arevalo-López, Quintin N. Meier, Claire Minaud, Marielle Huvé, Frédéric Capet, Andrés Cano, Robert Glaum and Olivier Mentré;
J. Am. Chem. Soc. (2024) online. https://doi.org/10.1021/jacs.4c070222

[185]
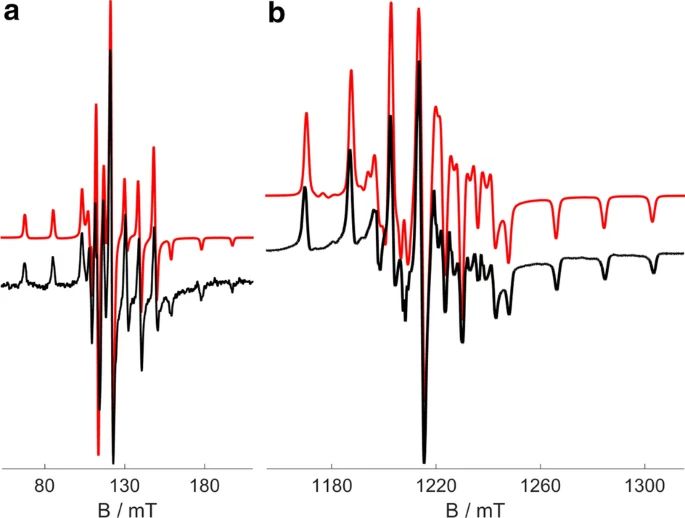
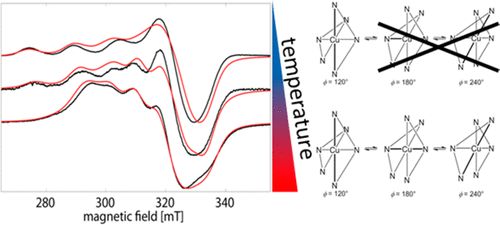
"Neutron diffraction, muon-spin rotation, and high magnetic field investigation of the multiferroic antiferromagnetic quantum spin-chain system CuCrO4"
J. M. Law, H. Luetkens, G. Pascua, Th. Hansen, R. Glaum, Z.-S. Wang, J. Wosnitza, and R. K. Kremer;
Phys. Rev. B 107 (2023) 184442. https://doi.org/10.1103/PhysRevB.107.1844426

[184]
"K3[MO4][MO3N] (M = Tc, Re) – Nitridooxorhenate and -technetate from highly alkaline media"
Désirée Badea, Selina Olthof, Jörg M. Neudörfl, Robert Glaum, Rainer Pöttgen, Maximilian Kai Reimann, Klaus Meerholz, Max Reimer, Christian Logemann, Erik Strub and Jörn Bruns;
European Journal Inorganic Chemistry (2023). https://doi.org/10.1002/ejic.2023001607

[182]
"Niobium-insertion into αII-VOPO4: Tuning catalytic properties for selective oxidation"
Frederik Rüther, Rhea Machado, Esteban Gioria, Sylvia L. Kunz, Knut Wittich, Patricia Löser, Michael Geske, Stephan A. Schunk, Robert Glaum, Frank Rosowski;
ACS Catalysis 13 (2023) 3295-3307. https://doi.org/10.1021/acscatal.2c062093

[180]
"An investigation on fatigue, fracture resistance, and color properties of aesthetic CAD/CAM monolithic ceramics"
Ahmed Mahmoud Fouda, Osama Atta, Mutlu Özcan, Bogna Stawarczyk, Robert Glaum, Christoph Bourauel;
Clin. Oral Invest. (2023). https://doi.org/10.1007/s00784-022-04833-y8

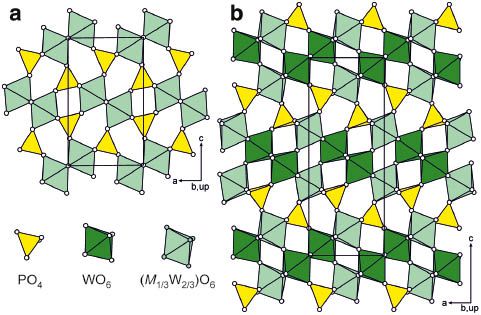
[179]
"Layered monophosphate tungsten bronzes [Ba(PO4)2]WmO3m–3: 2D-metals with locked charge-density-wave instabilities"
Hicham Nimoh, Angel M. Arevalo-López, Marielle Huvé, Claire Minaud, Andrés Cano, Robert Glaum and Olivier Mentré;
Angew. Chem. Int. Ed. (2023). https://doi.org/10.1002/anie.2023020499

[177]
"The weak ligand field in lanthanoid(III) hydrogensulfate-sulfates"
Sebastian Hein, Maximilian Jähnig, Nils Kannengießer, Jonathan Pape, Tobias Laporte, Gregor Schnakenburg, Reinhard K. Kremer, Werner Urland, and Robert Glaum;
Z. Anorg. Allg. Chem. 649 (2023). https://doi.org/10.1002/zaac.20220034210

[176]
"Platinum group metal phosphates as catalysts for selective C-H activation of lower alkanes"
Rhea Machado, Maria Dimitrakopoulou, Frank Girgsdies, Patricia Löser, Jingxiu Xie, Knut Wittich, Markus Weber, Michael Geske, Robert Glaum, Alexander Karbstein, Frank Rosowski, Sven Titlbach, Katarzyna Skorupska, Andrey V. Tarasov, Robert Schlögl, Stephan A. Schunk;
ACS Catalysis (2022) online accessible. https://doi.org/10.1021/acscatal.2c0264511

[174]
"Osmium(IV) pyrophosphate: Synthesis, Crystallization, and Ligand-Field Analysis of the [OsIVO6] Chromophore"
Patrick Rössel, Anke Wolfshohl, Jörg Daniels, Robert Glaum;
Z. Anorg. Allg. Chem. 648 (2022). https://doi.org/10.1002/zaac.20220001312

[172]
"Fertilizers"
Robert Glaum
Buchbeitrag „Applied Inorganic Chemistry“, Hrsg. T. Jüstel, R. Pöttgen, C. A. Strassert, De Gruyter Publ. (2022).
https://doi.org/10.1515/9783110798890-01413

[171]
"Phosphates"
Thomas Staffel, Robert Glaum
Buchbeitrag „Applied Inorganic Chemistry“, Hrsg. T. Jüstel, R. Pöttgen, C. A. Strassert, De Gruyter Publ. (2022).
https://doi.org/10.1515/9783110798890-01014

[170]
"Mixed-metal monophosphate tungsten bronzes containing rhodium and iridium"
A. Karbstein, M. Weber, D. Lahr, J. Daniels, W. Assenmacher, W. Mader, F. Rosowski, S.A. Schunk, R. Glaum;
Eur. J. Inorg. Chem. 2021. https://doi.org/10.1002/ejic.20210004715
Ältere Publikationen
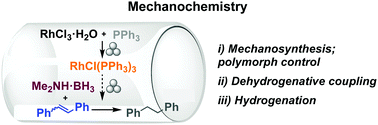
[159]
"Mechanochemical dehydrocoupling of dimethylamine borane and hydrogenation reactions using Wilkinson’s catalyst"
C. Schumacher, D.E. Crawford, B. Raguz, R. Glaum, S.L. James, C. Bolm and J.G. Hernandez;
Chem. Commun. 2018, 54, 8355-8358. https://doi.org/10.1039/C8CC04487B24
[158]
"Open-Shell 3d Transition Metal Nitridophosphates MIIP8N14 (MII=Fe, Co, Ni) by High-Pressure Metathesis"
S.D. Kloß, O. Janka, T. Block, R. Pöttgen, R. Glaum, and W. Schnick;
Angew. Chem. Int. Ed. 2019, 58, 4685–4689. http://dx.doi.org/10.1002/anie.20180914625
[157]
"New 2D and 3D Coordination Polymers by Dehydration of 1∞[MII(tF-BDC)(H2O)4] (MII= Zn2+, Co2+, Ni2+ and tF-BDC2-= Tetrafluoroterephthalate)"
C. Stastny, B. Dolfus, C.T. Brombach, D. Dresen, S. Disch, R. Glaum and U. Ruschewitz;
Z. Anorg. Allg. Chem. 2018, 644, 1423–1430. http://dx.doi.org/10.1002/zaac.20180022826
[156]
[155]
[154]
[153]
[152]
"BonnMag: Computer Program for Ligand-Field Analysis of fn Systems within the Angular Overlap Model"
A. Bronova , T. Bredow, R. Glaum, M.J. Riley and W. Urland;
Journal of Computational Chemistry 2018, 39, 176–186. http://dx.doi.org/10.1002/jcc.2509627
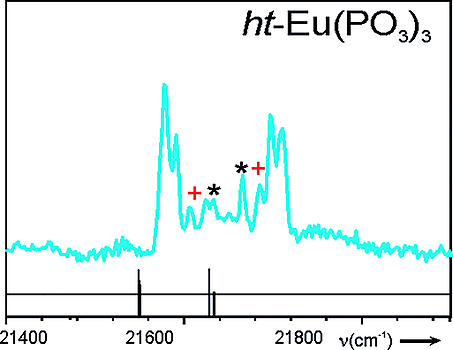
[151]
"Analysis of Ligand Field Effects in Europium(III) Phosphates"
R. Glaum, W. Grunwald, N. Kannengießer and A. Bronova;
Z. Anorg. Allg. Chem. 2020, 646, 184–192. http://dx.doi.org/10.1002/zaac.20200001928

[150]
"Synthesis and Characterization of the High-Pressure Nickel Borate γ‑NiB4O7"
M.K. Schmitt, O. Janka, O. Niehaus, T. Dresselhaus, R. Pöttgen, F. Pielnhofer, R. Weihrich, M. Krzhizhanovskaya, S. Filatov, R. Bubnova, L. Bayarjargal, B. Winkler, R. Glaum and H. Huppertz;
Inorg. Chem. 2017, 56, 4217−4228. https://doi.org/10.1021/acs.inorgchem.7b0024329
[149]
[148]
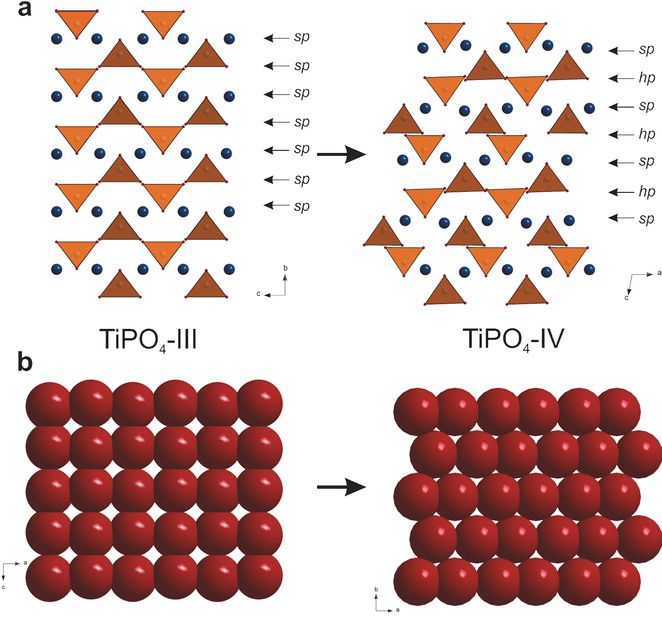
[147]
"High-Pressure Phase Transformations in TiPO4: A Route to a Pentacoordinate Phosphorus"
Maxim Bykov*, Elena Bykova, Michael Hanfland, Hanns-Peter Liermann, Reinhard Kremer, Robert Glaum, Leonid Dubrovinsky, Sander van Smaalen;
Angew. Chem. Intl. Ed. 55 (2016) 15053 - 15057. https://doi.org/10.1002/ange.20160853030
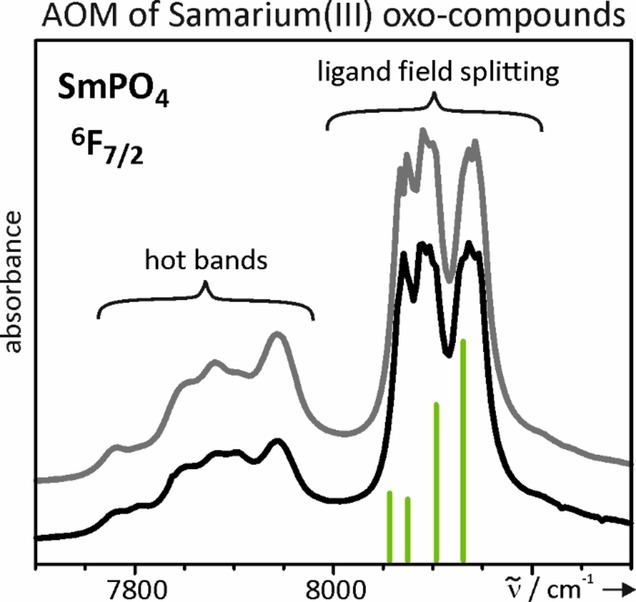
[146]
"Understanding optical absorption spectra and magnetic behavior of a wide range of samarium(III) oxo-compounds: Analysis of the ligand-field effects"
Nils Kannengießer, Maximilian Jähnig, Reinhard K. Kremer, and Robert Glaum;
Eur. J. Inorg. Chem. (2021). https://doi.org/10.1002/ejic.20200111531
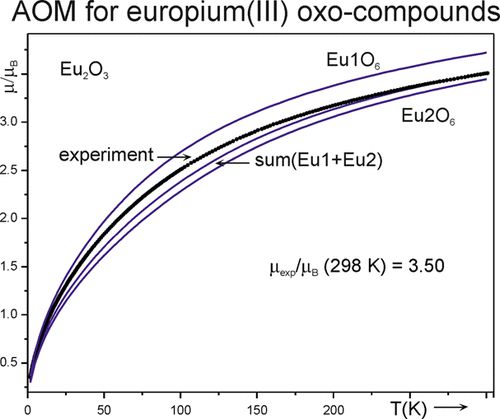
[145]
"Optical spectra and magnetic behavior of a wide range of europium(III) oxo-compounds: Analysis of the ligand-field effects"
Anna Bronova, Nils Kannengießer, and Robert Glaum;
Inorg. Chem. (2017) 56(15), 9235–9246. https://doi.org/10.1021/acs.inorgchem.7b0128732
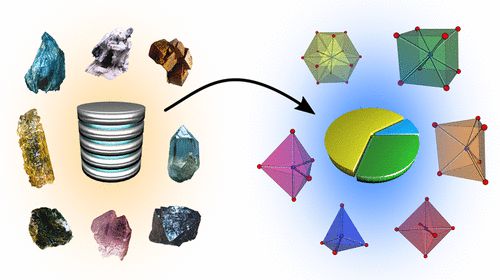
[144]
"Statistical analysis of coordination environments in oxides"
David Waroquiers, Xavier Gonze, Gian-Marco Rignanese, Cathrin Welker-Nieuwoudt, Frank Rosowski, Michael Göbel, Stephan Schenk, Peter Degelmann, Rute André, Robert Glaum, and Geoffroy Hautier;
Chem. Mater. (2017). https://doi.org/10.1021/acs.chemmater.7b0276633
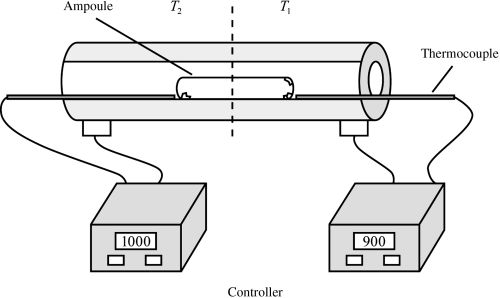
[143]
"Chemical Vapor Transport"
M. Binnewies, M. Schmidt, P. Schmidt and Robert Glaum;
in Handbook of Solid State Chemistry; Eds. Dronskowski, Kikkawa, Stein, Vol. 2 (2017) Wiley-VCH. https://doi.org/10.1002/9783527691036.hsscvol202034
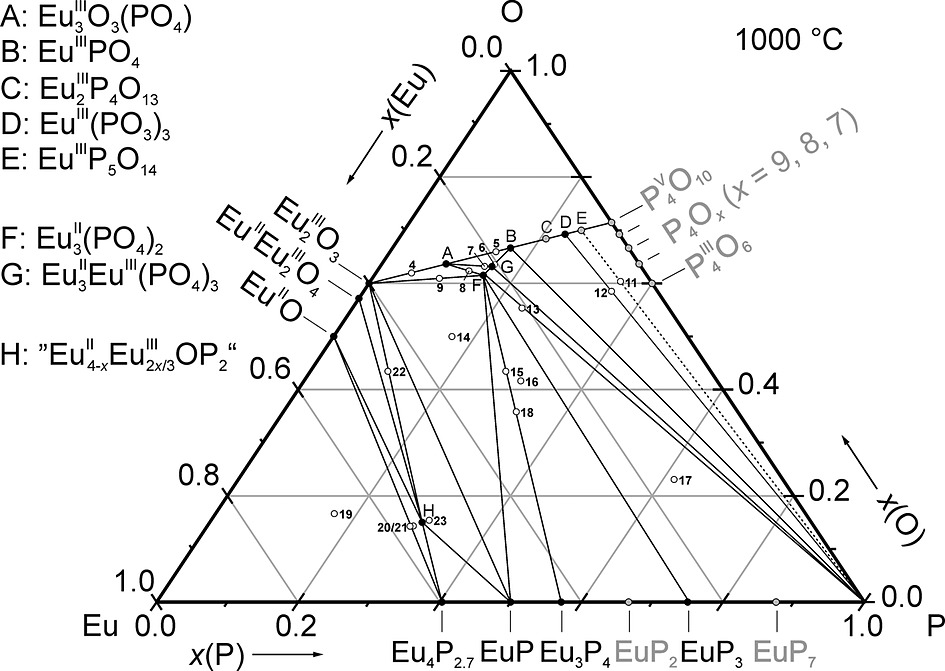
[142]
"Anhydrous europium phosphates: A comprehensive report on syntheses, crystal structures and phase relations"
Waldemar Grunwald, Knut Wittich and Robert Glaum;
Z. Anorg. Allg. Chem. (2018) 644, 1403-1414. https://doi.org/10.1002/zaac.20180019335
[141]
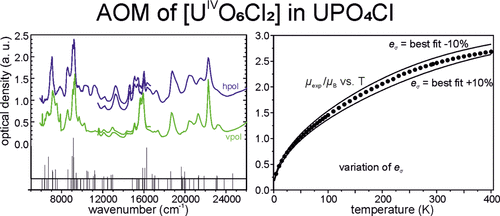
[140]
"The electronic states of U4+ in U(PO4)Cl - An example for angular overlap modeling of 5fn systems"
Anna Bronova, Thomas Bredow, Robert Glaum, and Werner Urland;
Inorg. Chem. (2016) 55, 6853-6860. https://doi.org/10.1021/acs.inorgchem.6b0036736
[139]
"Comprehensive characterization of the electronic structure of U4+ in uranium(IV) phosphate chloride"
Anna Bronova, Thomas Droß, Robert Glaum, Alexander Kostencki, Heiko Lueken, Achim Rhode, Manfred Speldrich, and Werner Urland;
Inorg. Chem. (2016) 55, 6848-6852. https://doi.org/10.1021/acs.inorgchem.6b0043837
[138]
"Substitution of W5+ in monophosphate tungsten bronzes by combinations Mn+/W6+"
Subrata Chandra Roy, Wilfried Assenmacher, Thomas Linden, Lars Esser,
Werner Mader, and Robert Glaum;
Z. Naturforsch. B (2016) 71, 543-552. https://doi.org/10.1515/znb-2016-003638

[137]
"Metastable solid solutions and equilibrium relations in the quasi-binary systems MoOPO4 – MOPO4 (M = V, W)"
Markus Weber, Subrata Chandra Roy, Yaser NejatyJahromi, Dinar Abdullin, Olav Schiemann, and Robert Glaum;
J. Solid State Chem. (2018) XX, XXXX-XXXX.

[136]
"Wolframphosphate der ReO3-Strukturfamilie"
Cathrin Welker-Nieuwoudt, Frank Rosowski, Michael Göbel, Robert Glaum, Subrata Chandra Roy, Geoffroy Hautier, David Waroquiers, Raoul Naumann D’Alnoncourt, Verena Strempel, Stephanie Linke;
Europäische Patentanmeldung 0000078320EP01 (2015) eingereicht am 01.07.2015.
[135]
"Bis-terpyridine copper(II) tetraphenylborate: An example for Jahn-Teller isomerism?"
Andreas Meyer, Gregor Schnakenburg, Robert Glaum, Olav Schiemann;
Inorg. Chem. (2015). https://doi.org/10.1021/acs.inorgchem.5b0115739
[134]
"Synthesis and Crystal Structure of Metal(III) Tungstenyl(VI) Orthophosphate Pyrophosphates"
Subrata Chandra Roy, Branimir Raguž, Wilfried Assenmacher, and Robert Glaum;
Solid State Sci. 49 (2015) 18-28. https://doi.org/10.1016/j.solidstatesciences.2015.09.00640
[133]
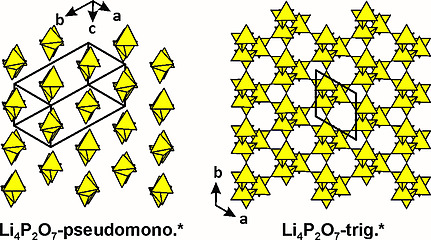
[132]
"Two new, metastable polymorphs of lithium pyrophosphate Li4P2O7"
B. Raguž, K. Wittich, R. Glaum;
Eur. J. Inorg. Chem. (2019) 1688-1696. https://doi.org/10.1002/ejic.20180110041
[131]
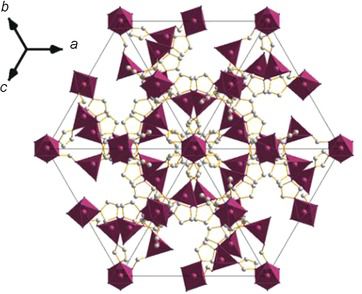
[130]
"Homonuclear Mixed-Valent Cobalt Imidazolate Framework for Oxygen-Evolution Electrocatalysis"
Erik A. Flügel, Vincent W.-H. Lau, Hendrik Schlomberg, Robert Glaum, and Bettina V. Lotsch;
Chem. Eur. J. 22 (2016). https://doi.org/10.1002/chem.20150415142
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
in Bearbeitung
Kontakt
Prof. Dr. Robert Glaum
Tel.: +49 228 73-5353
E-Mail: rglaum@uni-bonn.de
Raum: 1.030
Adresse
Institut für Anorganische Chemie
Gerhard-Domagk-Str. 1
53121 Bonn
Links
- https://doi.org/10.1002/zaac.201400160
- https://doi.org/10.1021/jacs.4c07022
- https://doi.org/10.1021/acscatal.2c06209
- https://doi.org/10.1002/adfm.202400054
- https://doi.org/10.1002/zaac.201300606
- https://doi.org/10.1103/PhysRevB.107.184442
- https://doi.org/10.1002/ejic.202300160
- https://doi.org/10.1007/s00784-022-04833-y
- https://doi.org/10.1002/anie.202302049
- https://doi.org/10.1002/zaac.202200342
- https://doi.org/10.1021/acscatal.2c02645
- https://doi.org/10.1002/zaac.202200013
- https://doi.org/10.1515/9783110798890-014
- https://doi.org/10.1515/9783110798890-010
- https://doi.org/10.1002/ejic.202100047
- https://doi.org/10.1007/s00723-020-01303-0
- https://doi.org/10.1002/chem.202003214
- https://doi.org/10.1039/D0CC01920H
- https://doi.org/10.1002/zaac.202200040
- https://doi.org/10.1103/PhysRevB.101.245106
- https://doi.org/10.1515/zkri-2020-0037
- https://doi.org/10.1515/znb-2019-0189
- https://doi.org/10.1021/acs.iecr.8b04328
- https://doi.org/10.1039/C8CC04487B
- http://dx.doi.org/10.1002/anie.201809146
- http://dx.doi.org/10.1002/zaac.201800228
- http://dx.doi.org/10.1002/jcc.25096
- http://dx.doi.org/10.1002/zaac.202000019
- https://doi.org/10.1021/acs.inorgchem.7b00243
- https://doi.org/10.1002/ange.201608530
- https://doi.org/10.1002/ejic.202001115
- https://doi.org/10.1021/acs.inorgchem.7b01287
- https://doi.org/10.1021/acs.chemmater.7b02766
- https://doi.org/10.1002/9783527691036.hsscvol2020
- https://doi.org/10.1002/zaac.201800193
- https://doi.org/10.1021/acs.inorgchem.6b00367
- https://doi.org/10.1021/acs.inorgchem.6b00438
- https://doi.org/10.1515/znb-2016-0036
- https://doi.org/10.1021/acs.inorgchem.5b01157
- https://doi.org/10.1016/j.solidstatesciences.2015.09.006
- https://doi.org/10.1002/ejic.201801100
- https://doi.org/10.1002/chem.201504151