Research
Research in the Filippou group focuses on the areas of modern molecular chemistry of the transition metals and main group elements. Emphasis is placed on the isolation and characterization of highly reactive closed- and open-shell compounds containing silicon, germanium, tin, and transition metals in novel bonding modes, and on the use of the compounds. Specialized synthetic as well as advanced analytical and quantum chemical methods are employed to achieve these goals.
Triple bonds of Si - Pb with transition metals
Multiple bonded compounds of the elements of the 2nd row of the periodic table are stable and ubiquitous in all areas of chemistry. For example alkenes and alkynes are known for more than 150 years and are central building blocks in the organic synthesis and the chemical industry due to the particularly rich and diverse chemistry of their π-bonds. In contrast, multiply bonded compounds of the p-block elements of the higher periods (n > 3) were considered for a long time unstable molecules. This phenomenon was rationalized assuming that these elements cannot form stable (p-p)π-multiple bonds because of the increasing difference in the radii of the ns and np valence orbitals preventing an isovalent orbital mixing (hybridization). Since the first report on stable ditetrelenes about 35 years ago, experimental and quantum chemical studies on multiple bonds of the heavier group 14 elements (Si - Pb) have attracted great interest, since these compounds exhibit significant structural and electronic differences compared to the carbon analogues, leading to completely new chemical properties.
During the past 15 years we have been exploring the chemistry of tetrelylidyne complexes of the general formula LnM≡ER, where M is a transition metal, Ln is a specially tailored ligand sphere, E is one of the heavier tetrels (Si - Pb), and R is a bulky organyl substituent. Due to the high polarity of the M≡E triple bond, the chemistry of these novel, highly reactive compounds differs markedly from that of their carbon analogues, the transition metal-carbyne complexes, which represent an important class of organometallic compounds with numerous applications in catalysis. Current studies focus on the unusual reactivity of these compounds providing access to unprecedented materials, e. g. compounds of planar-tetracoordinated silicon or coordination compounds with novel Si-based ligands.


Molecular silicon chemistry in low oxidation states
Due to its long natural abundance and its special properties, silicon is an important element that is used in many materials of great technological importance. Various silicon compounds in low oxidation states are important species in chemical vapor deposition for the production of semiconductors and solar cells or in the synthesis of organochlorosilanes in the Mueller-Rochow process. In our research group, we try to tame these highly reactive species and explore their chemistry under normal laboratory conditions.
Recent developments in molecular silicon chemistry have shown that N-heterocyclic carbenes (NHCs) are capable of stabilizing silicon centers at unusually low oxidation states and novel bonding modes. Interesting examples include the disilicon (0) compound (NHC)Si=Si(NHC), the Si(I) halides Si2X2(NHC)2 (X = Cl, Br, I), and the Si(II) halides SiX2 (NHC) (X = Cl, Br, I), which have proven to be particularly useful building blocks in low-oxidation state silicon chemistry.
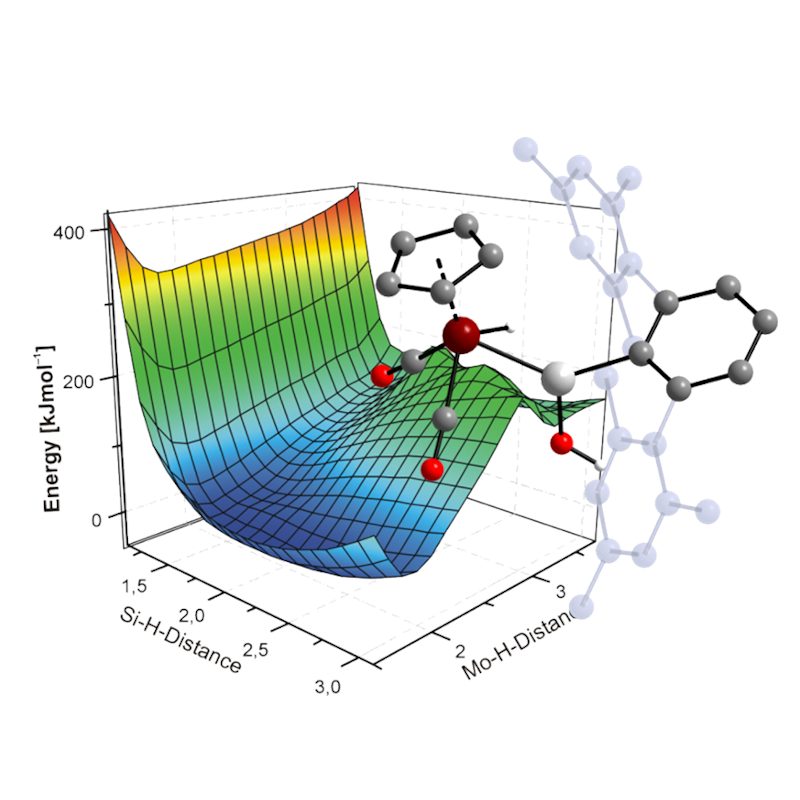
Computational Chemistry
We apply a wide range of different ab-initio and DFT-based quantum chemical methods, as well as methods of analysis of the wavefuntion of the novel compounds prepared in the group. This allows to gain deeper insight into the electronic structure of the Se compounds, leading to a better understanding of their chemical properties and predict their reactivitiy. As next, the reaction pathways are studied to rationalize the outcome of reactions.
Initial research in our group frocused on understanding the electronic structure and the reactivity of compounds featuring M≡E triple bonds (M = Mo, W; E = Si, Ge, Sn, Pb). More recently, studies have been extended to a wide range of novel compounds of silicon and germanium featuring unusual electronic molecular geometries and elevtronic structures.